
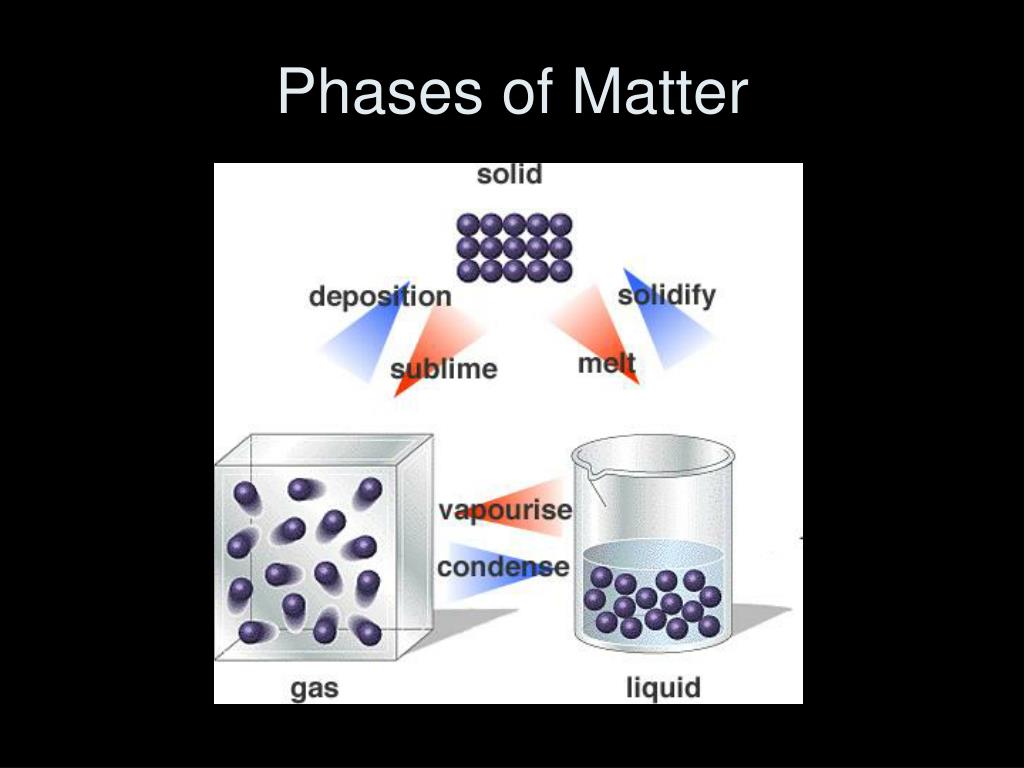
There is not much space between the particles, but they can slide past each other and flow easily. Liquids have a fixed volume, but take the shape of the container in which they sit.

There is not much space between the particles and there is little particle movement. A solid’s particles are packed closely together. Less commonly, we can also find matter as plasma or Bose-Einstein (BE) condensate. The three most common phases of matter on Earth are solids, liquids and gases. Matter can be found in several phases or states. Matter is something that has mass and volume (takes up space).
#STATES OF MATTER AND PHASE CHANGES FREE#
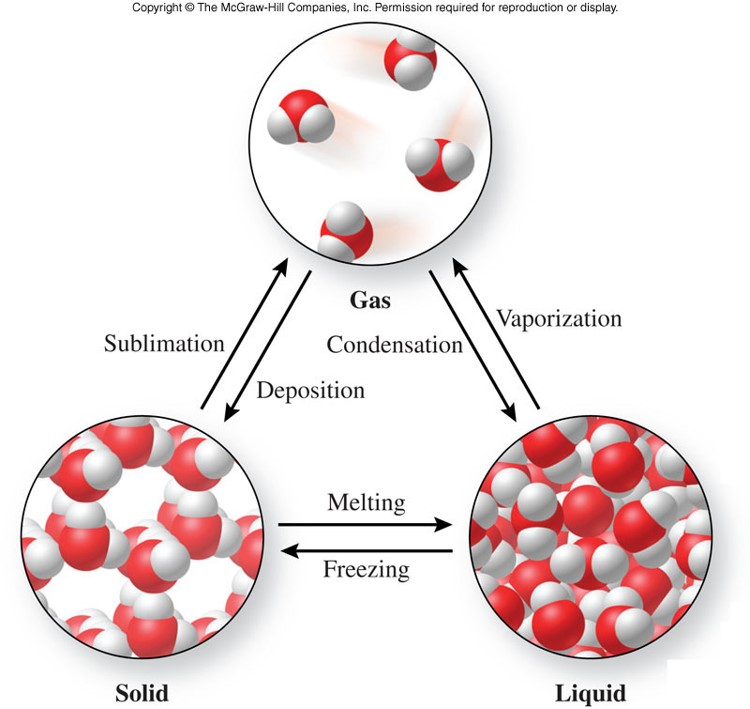
A free teacher account also allows you to create playlists of games and assignments for students and track class progress.2) Name the three phases (states) of matter?ģ) Identify and describe the particle in each phase of matter and how they are different in each phase of matter. You can access all of the games on Legends of Learning for free, forever, with a teacher account. For some types of rock, decreasing pressure can also cause them to melt.Ī preview of each game in the learning objective is found below. Decreasing pressure can cause it to vaporize. When the pressure exerted on a substance increases, it can cause the substance to condense. When energy is removed, the opposite happens, decreasing the substance’s temperature and turning it from liquid to solid (freezing), gas to solid (deposition), or from gas to liquid (condensation). When thermal energy is added to a substance, its temperature increases, which can change its state from solid to liquid (melting), liquid to gas (vaporization), or solid to gas (sublimation). Both temperature and pressure can be measured, and state changes can be observed. Physical conditions like temperature and pressure affect state of matter. Gases expand or contract to fill the available space, meaning they don’t maintain their shape or volume.Ī substance’s state of matter is an extrinsic property, meaning it can be changed by its environment. They move quickly relative to each other.

Particles are in constant motion, but they interact differently depending on the state of matter. A substance’s state of matter - solid, liquid, gas, or plasma - depends on how its molecules move and maintain their volume and shape. Concepts CoveredĪtoms and molecules are the particles that make up matter. Scroll down for a preview of this learning objective’s games and the concepts they drive home. The Effects of Temperature and Pressure on State learning objective - based on NGSS and state standards - delivers improved student engagement and academic performance in your classroom, as demonstrated by research.
#STATES OF MATTER AND PHASE CHANGES SERIES#
In this series of games, your students will learn how and why substances undergo phase changes.


 0 kommentar(er)
0 kommentar(er)
